By: Felistas Kumwenda-Mwakiseghile, TyVAC Trial Coordinator, Malawi Liverpool Wellcome Trust & Fleesie Hubbard, M.S., International Regulatory Affairs Specialist, University of Maryland, Baltimore, Center for Vaccine Development & Global Health.
Behind every vaccine study are regulatory processes to ensure the protection of participants and researchers. There are seven basic regulatory forms required for a research study:

[University of Maryland, Baltimore] [Natalie Fecteau]
Study protocols are submitted to an Institutional Review Board (IRB) that reviews and monitors biomedical research involving human subjects. The purpose of an IRB is to ensure researchers follow the study protocol and that research participants are protected.
For international studies with multiple partners, each institution submits the protocol to its individual IRB, or ethics committee. For example, the TyVAC protocol in Malawi was submitted to the Malawi Ministry of Health, National Health Sciences Research Committee (NHSRC), Pharmacy, Medicines & Regulatory Authority, University of Maryland, Baltimore IRB, University of Liverpool, and Institutional Biosafety Committee. When there are multiple IRBs for one study, all IRBs must agree to a federal-wide assurance (FWA), which is documentation of an institution’s commitment to comply with Federal regulations and maintain policies and procedures for the protection of human participants. There are multiple regulatory procedures specific to vaccine studies, such as import and export permits for vaccine shipment, pharmacy board review, safety and stability testing of vaccines, and regular monitoring.
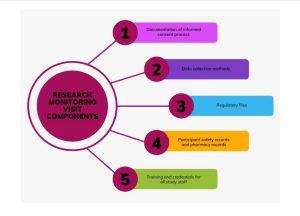
Studies involving vaccines are more closely monitored and the frequency of monitoring is determined by the complexity of the study. For example, TyVAC is monitored quarterly – at a minimum – by the Malawi-Liverpool-Wellcome Programme Clinical Research Support Unit and annually by the study sponsor and/or a contracted external Clinical Research Organization. This process is in place to verify that data collected are authentic, accurate, and complete and to ensure that the safety and rights of subjects are protected during the study. The monitoring visit consists of reviewing the informed consent process, data collection methods, regulatory files, participant safety records, and staff training credentials, as shown below:
[University of Maryland, Baltimore] [Natalie Fecteau]
Monitoring occurs on an ongoing basis for various elements of the research study, such as ensuring safe transport of vaccines and samples. Additionally, there are regular monitoring visits to confirm that all aspects of the protocol are being followed correctly. Annually, the local ethics committee in Malawi (NHSRC) and University of Maryland, Baltimore IRB require a continuing review of the protocol. The research team submits a general overview of study progress, including number of participants enrolled, any reports by the Data Safety Monitoring Board, interim analysis of data, and plans for the upcoming year. At the end of a study, a closure report is submitted to all IRBs describing the final study results.
The informed consent process protects research participants by fully informing them of the study’s risks and benefits. Informed consent details every aspect of the study to allow potential participants to choose whether to participate. If there are changes that require an amendment, a change in the study protocol and/or informed consent documents is drafted as an amendment and submitted for review to the IRB. In some cases, participants must be re-consented with an updated consent form reflecting the change. For example, if an additional interview or clinical visit is added to a long-term study, participants decide if they want to continue with the study. Research participants should be continuously informed about the study objectives, procedures, and risks/benefits of participating.
The regulatory process ensures accurate data collection, allowing the data to be analyzed rigorously and results published. The TyVAC studies have added evidence-based knowledge that is critical to informing decision-making. Regulatory processes, as in Malawi, demonstrate that a study has a high level of rigor and meets standards of scientific research. These processes provide the framework for research studies to contribute to scientific knowledge. A valuable result of such research is bringing vaccines to the world and in the case of TyVAC, Taking on Typhoid.